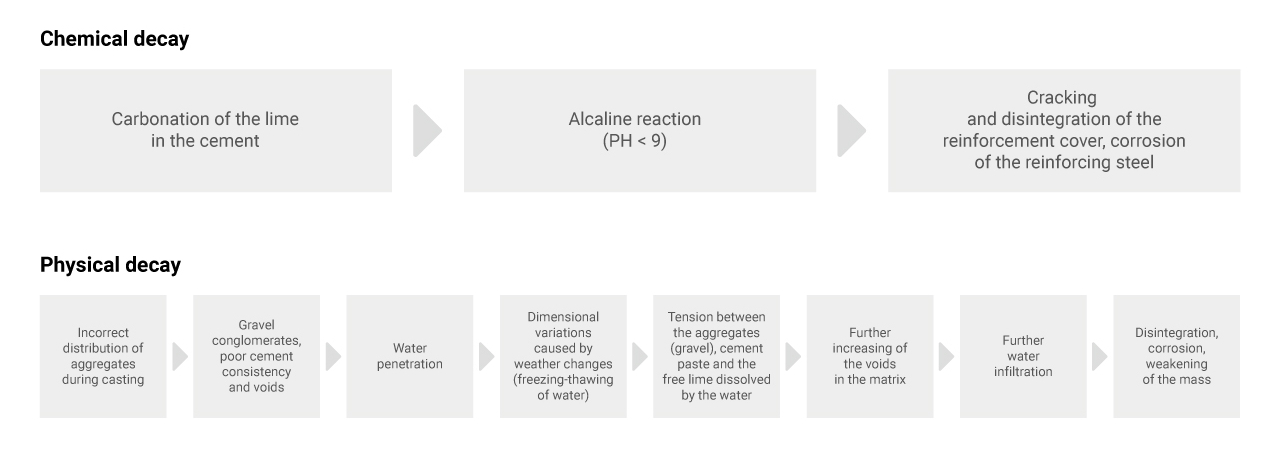
The main hazard facing reinforced concrete is chemical: carbonation of the lime. This phenomenon is caused by the carbon dioxide and sulphur dioxide in the air and water, products that react with the lime released by the concrete to form insoluble calcium carbonate crystals. The resulting alkaline reaction (to a pH of below 9) facilitates erosion of the passivating layer of the reinforcing steel, thus leading to oxidation, increased volume, swelling and disintegration which cause various pathologies: cracking of the concrete; detachment of reinforcement cover; disintegration of the concrete; corrosion of the reinforcing steel. Besides chemical phenomena, reinforced concrete is subject to strong physical decay. One typical example is the presence of “gravel nests” caused by incorrect distribution of the aggregates when casting the formworks. The cement consistency of these agglomerates is poor and they present voids which subject the reinforced concrete to rapid decay. Dimensional variations due to changes in the weather (freezing-thawing of the water contained in the pores in the concrete) generate tensions between the aggregates and the cement paste, while the water solubilizes the free lime, increasing the voids in the matrix which, in turn, means further infiltration, corrosion and weakening of the mass.



 Ivas Industria Vernici Spa
Ivas Industria Vernici Spa